Steel is one of the world’s most used materials, made from a combination of iron and carbon, and various other elements. From the smallest construction to the most complex structures, it is everywhere surrounding and impacting us daily.
As it plays such a vital role, knowledge about this material is pretty uncommon.
What is Steel?
Steel has played a vital role in the development of modern civilization and is not to be confused with iron. In the simplest form, Steel is iron ore alloyed with less than 2% carbon.
However, many other elements like Chromium, Aluminum, Manganese, Iron, Phosphorus, Sulfur, Copper, Nickel, etc. can also be added to produce several grades of steel alloys with different properties.
Steel has many advantages in the construction industry, which include many parameters like tensile strength, compressive strength, yield strength, ultimate strength, fatigue strength, elongation, ductility, low weight, hardness, resistance to corrosion, resistance to fire, etc.
Depending on the many parameters steel can be classified into the following types:
1. Stainless steel
2. Carbon steel
3. Tool steel
4. Alloy steel
Effect of Sulfur Content on Steel
Sulfur(s), an element is always present in small amounts in steel, and its removal during steelmaking is a tedious and challenging method.
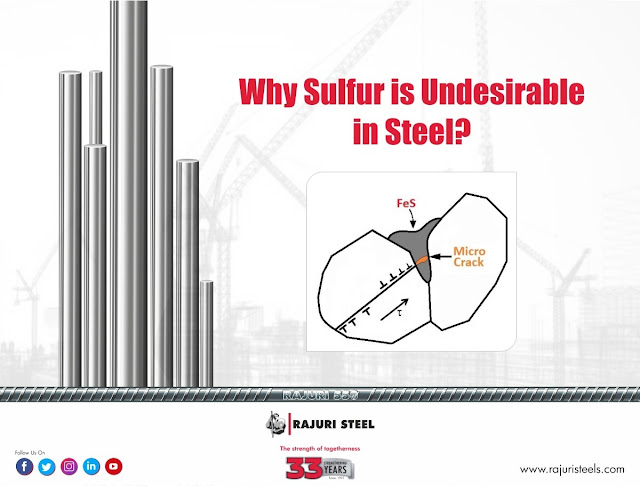
Sulfur(s), whose atomic number is 16 and atomic weight is 32.066, is usually an undesirable impurity in the steel rather than an alloying element. During the solidification process, it can react with iron(Fe) to form iron sulfide (FeS) at the grain boundaries. Formation of FeS can develop areas of weakness in the material, which are more sensitive to cracking & can lead to the building of hot cracks in the steel.
Sulfur(s) enhances machinability but decreases transverse ductility and notched impact toughness, and has a nominal effect on longitudinal mechanical properties.
The Sulfur(s) content of steel in amounts exceeding 0.05%, tends to cause brittleness and reduce weldability. Alloying additions of Sulfur(s) in amounts from 0.10 to 0.30% will tend to enhance the machinability of the steel. Such alloys are referred to as 'resulphurized' or 'free-machining'. Free-machining alloys are not intended for use where welding is needed.
Steel grades have varying sulfur content, however, in most cases, the high Sulfur(s)content in steel creates a negative impact on properties, which include:
1. Formation of crack because of sulfides which leads to fatigue failure
2. Embrittlement of steel and reduction of weldability, especially when sulfur content is more than 0.05%
3. Reduction of intergranular strength and the melting point of the steel.
These are the reason why "desulphurization" technologies are increasingly exercised to deliver high-quality steel components for industries. On the other hand, when the steel is too low in sulfur, it can create difficulties in weldability. Reduced Sulfur(s) can lead to a negative impact on the fluid flow and lead to a wide weld pool with shallow penetration.
If the steel has a high Sulfur(s) but is low in Manganese (Mn), it produces the most prominent segregation of all steel accompanying components. It leads to red or hot shortness. In the absence of Manganese (Mn), Sulfur(s) readily incorporates with iron (Fe) to form low-melting iron sulfide(FeS). In regular steels, even in re-sulphurized, Mn contents are high sufficiently (Mn/S = 8 minimum) to prevent extreme hot shortness during rolling or forging. Hence, Sulfur(s) occurs in the microstructure as MnS inclusions.
When it comes to sulfide inclusions in steel, fatigue failure is a common failure, and is controlled by crack nucleation and growth.
The inclusion of sulfide (FeS) reduces weldability and corrosion resistance. While reheating, the presence of Sulfur(s) may also lead to tears and cracks. When Sulfur(s) content increases in the steel the weldability decreases and that's the reason the high Sulfur(s) content steel has poor welding properties.



Comments
Post a Comment